How Many Bonds Can Hydrogen Make
Water molecule can haveform a maximum of four hydrogen bonds. It can only form one ionic bond or covalent bond.

Question Video Stating How Many Hydrogen Bonds Can Be Formed By A Single Water Molecule Nagwa
How many hydrogen bonds can a single water molecule form quizlet.

. The number refers to the number of bonds each of the element makes. That molecule is involved in 4 hydrogen bonds. But if you take 100 water molecules and count how many hydrogen bonds there are between them the answer will be about 200 because each molecules makes 2 bonds.
Its called the HONC rule or sometimes known as HONC 1234 rule. Hydrogen bonding occurs only in molecules where hydrogen is covalently bonded to one of three elements. The strength of a typical hydrogen bond is about 5 of that of a covalent bond.
These three elements are so electronegative that they withdraw the majority of the electron density in the covalent bond with hydrogen leaving the H atom very. Each hydrogen can form one bond with selenium. Two given through the H atoms towards two other H2O molecules and two received on the O atom from H atoms of two other H2O molecules.
Hydrogen forms 1 single bond when there is a s-s overlap which is the greatest overlap but it can also form 2 single bond where there are 2 s-p overlaps such as is Even in compounds like B X 2 H X 6 H seems to make 2 bonds but it doesnt. An alcohol is an organic molecule containing an -OH group. Hydrogen makes 1 bond Oxygen makes 2 bonds Nitrogen makes 3 bonds and Carbon makes 4 bonds.
Each water molecule can form 4 hydrogen bonds. Hydrogen bonding in alcohols. Hydrogen is in its diatomic form H2 a component of our atmosphere and one.
If you consider each molecule making 4 bonds then you are double counting each bond being made and accepted. Fluorine oxygen or nitrogen. Hydrogen bonds with hydrogen bond acceptor atoms such as Oxygen.
Each selenium atom can form two bonds one with each hydrogen 2 hydrogen atoms total. Hydrogens electronegativity is also relatively average 224 for nonmetals and metals which means that it normally forms both polar and nonpolar covalent bonds. What Type of bond can hydrogen atom form.
Hydrogen bonds also occur when hydrogen is bonded to fluorine but the HF group does not appear in other molecules. Any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. This is because Hydrogen only has one electron surrounding its nucleus and its valence shell can only hold 2 electrons thus only allowing space for one bond.
These four elements are widely used when it comes to drawing Lewis structures at introductory chemistry level. As you can see the two Hydrogen atoms in the centre make 2 bonds each with Boron. Each selenium atom can form two bonds one with each hydrogen 2hydrogen atoms total.

Pin By 규뀨 On Hydrogen In 2021 Molecular Electrons Classroom

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

The Hydrogen Atom Has A Positive Charge Well The Oxygen Atom Has A Negative Charge Making The Compound A Covalent Bond And Covalent Bonding Chemistry Molecules

How To Predict Number Of Bonds Each Element Forms Chemsimplified

Chemical Bond Read Physical Science Ck 12 Foundation Chemical Bond Molecules Water Molecule

Hydrogen Bonds Vs Van Der Waals Hydrogen Bond Bond Chemistry

Chemistry Covalent Bonding Lesson Activities Covalent Bonding Chemistry Lessons Lessons Activities

Bonds Ionic Covalent Hydrogen Bonds Ionic Transfer Electrons Form Between Ions Covalent Share Electrons Weaker Than Covalent Bonding Ionic Bonding Bond

What Is The Bond Between Carbon And Hydrogen Quora

H 2 Hydrogen Gas Covalent Bond Bonds In Biology Weak Bonds Hydrogen Bonds Attraction Between And Hydrogen Bond Covalent Bonding Chemistry Basics

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

Hydrogen Bonds In Water Article Khan Academy

Carbon Chemistry Carbon Atoms Can Form Single Double Or Triple Bonds With Other Carbon Atoms Carbon Can Form Up To 4 Bonds This A Atom Carbon Cycle Chemistry

Reading Covalent Bonds Biology I
8 Atoms Tend To Form Bonds Until Their Valence Electron Shell Is Filled Labxchange

Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency Index Chemistry Counting By 2 S

Difference Between Covalent Bonding Hydrogen Bond Chemical Bond

Pattern Of Hydrogen Bonding In Water And Ice Hydrogen Bond Water Molecule Bond
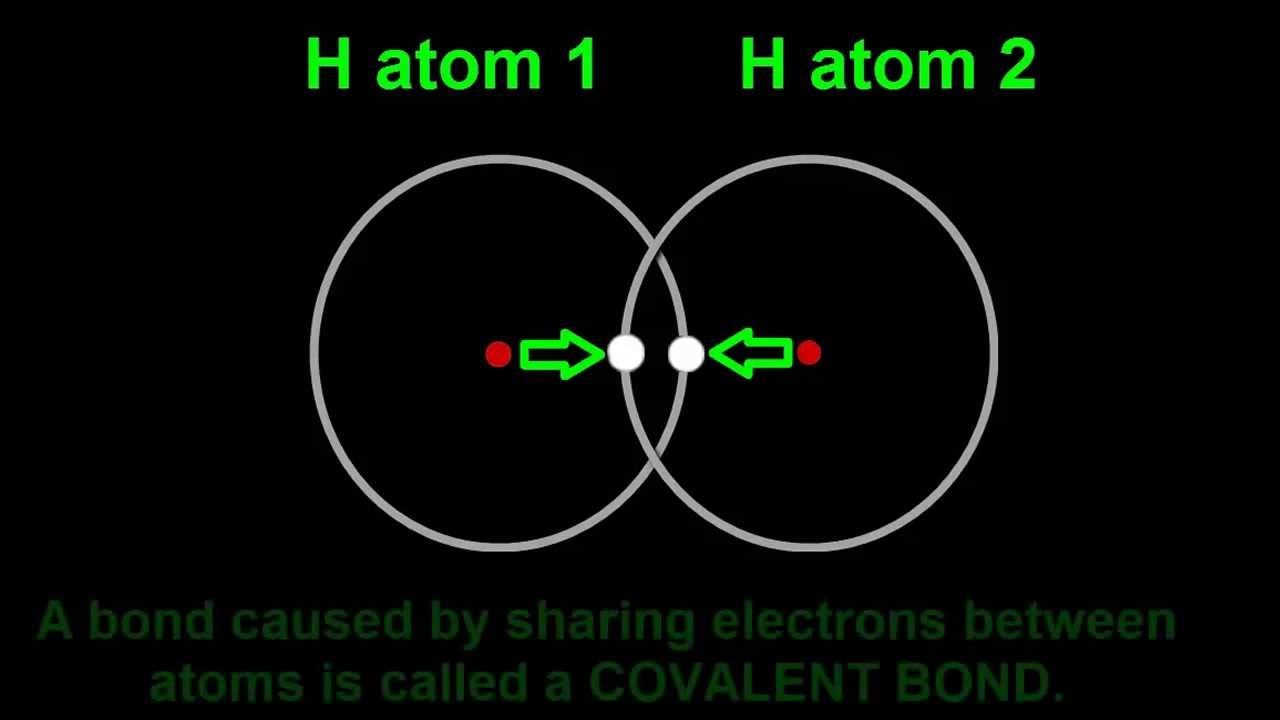
How Two Hydrogen Atoms Join To Become A Hydrogen Molecule H2 Covalent Bonding Molecules Hydrogen Atom
Comments
Post a Comment